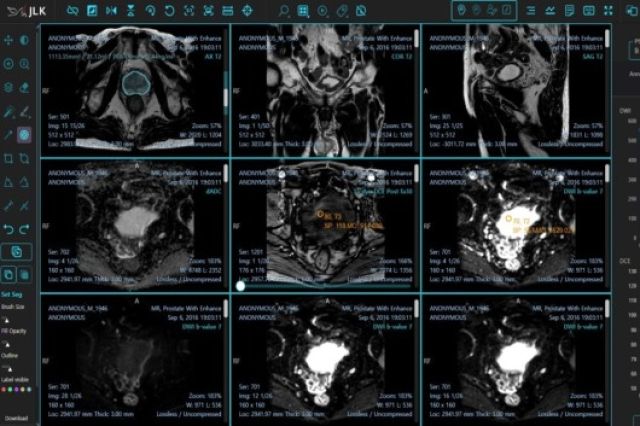
South Korea’s JLK Inc., an AI-based diagnosis solution and platform maker, announced on Monday that it got approval from the US Food and Drug Administration (FDA) for its prostate cancer diagnostic solution Medihub Prostate.
Medihub Prostate is an AI solution for prostate cancer diagnosis developed by JLK in collaboration with Seoul Asan Hospital and the University of Missouri in the US through clinical trials.
The solution aids prostate cancer diagnosis by analyzing prostate magnetic resonance (MR) images and assessing prostate-specific antigen (PSA) levels.
Prostate cancer is the most common cancer among men in the US, with a latent prostate cancer occurrence risk of up to 40%.
"Based on the FDA approval of MEDIHUB Prostate, we plan to pursue entry into the US market more aggressively," JLK's CEO Kim Dong-min said.
"We intend to sequentially apply for FDA approval for three additional AI solutions between August and October."
Write to Jeong Min Nam at
peux@hankyung.com